Diagnostic Criteria for Congenital Cerebral Hypomyelination
1. Definition and Disease Classification
Congenital cerebral hypomyelination constitute a group of diseases in which the myelin formation in the white matter of the cerebrum and other parts of the central nervous system is congenitally insufficient (hypoplastic) due to genetic factors. The diseases in this family are caused by genetic abnormalities that affect the structural components of myelin as well as factors required for myelination. They are characterized by widespread, severe reduction or cessation of myelination of the central nervous system. Thus far, eleven such diseases have been identified, including Pelizaeus-Merzbacher disease (PMD) (Table 1); this excludes demyelinating diseases and secondary dysmyelination associated with metabolic or systemic disorders.
2. Clinical symptoms and test results common to hypomyelination diseases
Congenital cerebral hypomyelination is associated with the presentation of the following two signs: (1) spastic quadriplegia (paraplegia) suggesting pyramidal tract involvement, and (2) diffuse hyperintense regions in the white matter as revealed by magnetic resonance imaging (MRI). Signs of demyelination are excluded. In addition, the following signs may also be present: (1) nystagmus, (2) psychomotor retardation, (3) cerebellar deficits such as ataxic symptoms in the trunk and limbs, intention tremor, dysmetria, dysdiadochokinesia, and dysarthria during childhood, (4) basal ganglion disorders such as rigidity and dystonia, (5) epilepsy, and (6) electrophysiological signs such as impaired central conduction in evoked potentials.
Some characteristics of these clinical symptoms have been described below. While pyramidal tract involvement is most often evident during infancy as hypotonia, tendon reflexes in the arms and legs, the Babinski reflex, spastic quadriplegia and other pathological reflexes gradually appear, increase and persist through adulthood. Mild cases of congenital cerebral hypomeylination have been known to exist with simple to complex forms of spastic paraplegia, which initially presents as leg spasticity. Psychomotor developmental retardation is present in almost all cases. In general, motor disabilities are more serious than mental disabilities, thus, affected individuals have better language comprehension as compared to their expressive language ability. Cerebellar deficits occur via both the afferent and efferent pathways and can take a variety of forms, including ataxic symptoms in the trunk and limbs, intention tremor, dysmetria, dysdiadochokinesia dysarthria and other symptoms during childhood. In contrast, basal ganglion symptoms such as rigidity and dystonia often appear with the passage of time. White matter abnormalities may be further complicated by cortical epilepsy. Neurophysiological tests show impaired central conduction in the auditory-brainstem response as well as the somatosensory and visual evoked potentials. Patients who exhibit peripheral neuropathy as revealed by peripheral nerve conduction velocity tests and electromyography are classified as type I, and those who do not as type II. MRI is considered highly useful for imaging-based diagnosis; diffuse hyperintensity of the white matter is evident on T2-weighted imaging. While hypointense areas of the white matter may be apparent on computed tomography (CT) images as well, this offers little diagnostic value. Serological and biochemical tests reveal no specific findings, with the exception in the case of Allan-Herndon-Dudley syndrome (AHDS).
3. Flowchart for differential diagnosis (Figure 1)
The first step in the differential diagnosis is to determine whether the hyperintensity on T2-weighted MRI represents demyelination or just the delay or cessation in regular myelination. In the event of mildly delayed myelination, tests are repeated after at least six months have elapsed, to determine whether or not myelination is retarded. In demyelinating diseases, severe hyperintensity is usually evident on T2-weighted images, the same areas appearing hypointense on T1-weighted MR images. Knowledge of the myelination pattern considered normal during childhood is essential in order to accurately judge whether or not myelination is delayed. Based on these criteria, patients are broadly divided into type I and type II, depending on whether their peripheral nerve conduction velocity measurements demonstrate the absence or presence of peripheral neuropathy, respectively. Table 1 lists the diseases in each category.
The next consideration in the differential diagnosis is that of genetic testing. Clinical data such as family history, the sex of the index case, age of onset and nystagmus are important. For example, if multiple deformities are present in type I-affected individuals, 18q-deletion syndrome is initially suspected, following which chromosome tests and 18q-subtelomeric fluorescence in-situ hybridization (FISH) are performed. If affected males exhibit thyroid dysfunction, AHDS is suspected. On the other hand, for male individuals with congenital cerebral hypomyelination without the abnormalities mentioned above, PMD is suspected as it is the most common of these conditions, following which, the patients are screened for PLP1 genetic defects. For affected male individuals with no apparent PLP1 defect, and affected female individuals, Pelizaeus-Merzbacher-like disease (PMLD) is suspected and the patients are screened for GJC2 gene defects. In absence of any GJC2 defects in these individuals, either possible Salla disease (SD), mitochondrial heat-shock protein 60 chaperonopathy (MitCHAP60), or hypomyelination with atrophy of the basal ganglia and cerebellum (HABC) is suspected. Individuals with SD exhibit high levels of free sialic acid in urine and cerebrospinal fluid samples, those with HABC show atrophy of the basal ganglia and cerebellum, and those with hypomyelination with cerebellar atrophy and hypoplasiaof the corpus callosum (HCAHC) show atrophy of the cerebellum and hypoplasia of the corpus callosum without atrophy of the basal ganglia. The possibility of PMD or PMLD1 caused by rare mutations difficult to detect using conventional genetic analysis techniques, such as the partial duplication of PLP1 or mutations in the UTRs of PLP1 or GJC2, must also be considered.
Type II-affected individuals are comparatively easy to diagnose on the basis of the characteristics of their associated symptoms. For example, if cataract is present, they may suffer from hypomyelination and congenital cataract (HCC); if hypodontia or impaired growth is present, ataxia, delayed dentition, and hypomyelination (ADDH) are concerned; while in the event of Waardenburg syndrome or Hirschsprung disease, peripheral demyelinating neuropathy, central dysmyelinating leukodystrophy, Waardenburg syndrome, and Hirschsprung disease (PCWH) are considered. Individuals with PMD caused by a PLP1 loss-of-function mutation exhibit peripheral neuropathy.
Some, albeit few, affected individuals do not fit into any of the disease entities described by these classification criteria, suggesting that there may be some forms of congenital cerebral hypomyelination for which disease concepts have yet to be established.
4. Descriptions of different diseases
Type I: Congenital hypomyelination mainly affecting the central nervous system
(1) Pelizaeus-Merzbacher disease (PMD, HLD1, OMIM#312080)
Disease concept
As this form of hypomyelination of the central nervous system is transmitted by X-linked recessive inheritance, it only occurs in males, barring exceptional cases. Neuropathologically, it is characterized by the fact that although the myelination of the white matter of the central nervous system is lost or diminished, the neurons and axons are preserved with no obvious signs of demyelination, and the remaining myelin sheaths exhibit a patchy tigroid appearance. Genetically, the main cause of this disease is a defect in the proteolipid protein 1 (PLP1) gene.1
Clinical symptoms
Nystagmus is usually noticeable immediately after birth, latest by around 1 month of age. In most cases, it takes the form of horizontal nystagmus; however, compensatory head tremors may also be evident. Hypotonic symptoms start to appear between birth and approximately 6 months of age. The loss of primitive reflexes is delayed, with the Babinski reflex still present even after 6 months; eventually, increased tendon reflexes also appear, indicating problems with primary neurons. Close observation may reveal cerebellar symptoms in the form of intention tremor after about 1 year. Athetoid, abnormal limb positioning also begins to appear at 2 years of age. The successive appearance of symptoms of the central nervous system, motor, motor control system, and basal ganglia is characteristic of this disease. Nystagmus becomes less obvious later, while as joint contracture progresses, the cerebellar symptoms also become less noticeable, so that many children with spasticity and rigidity at age 5 are diagnosed with cerebral palsy. In some cases, children may exhibit a phenotype of X-linked spastic paraplegia, in which motor and intellectual development progress normally during infancy and early childhood, but then regress slowly after they start school. The symptoms of regression usually appear after their teens or twenties, and their average life expectancy is approximately 30 years. Imaging reveals cerebral atrophy that parallels this symptomatic regression. Individuals with a PLP1 loss-of-function mutation exhibit peripheral neuropathy.
Test results and imaging findings
Test results show loss of the auditory brainstem response, in that waves II–IV reflect patchy central somatosensory conduction time. Although peripheral nerve conduction velocity is normal, it may be slightly reduced in affected individuals with a PLP1 deletion or other loss-of-function mutations. Serum biochemistry and urinalysis test results do not indicate any abnormal patterns. Similarly, cerebrospinal fluid tests do not reveal obvious abnormalities including elevated protein. Unlike demyelinating diseases, the level of myelin basic protein is usually either within normal limits or at the upper limit of normal. However, MRI findings are highly characteristic, with distinctive diffuse white matter hyperintensity visible on T2-weighted imaging and hypointensity in the cortex and white matter seen on T1-weighted imaging. In general, the signal on T2-weighted imaging shows poorer myelination than that of a normal neonate, which changes little with increasing age. T1-weighted imaging reveals slower myelination, albeit with poor contrast in the cortex and white matter. CT only shows non-specific cerebral atrophy, and provides little diagnostic value.
Genetic diagnosis
A range of genetic defects may be present, including duplications, point mutations, and deletions in the PLP1 gene.1 PLP1 duplication is present in approximately half (50%–75%) of the cases. This is confirmed by using quantitative polymerase chain reaction (PCR) or interphase nucleus FISH to identify the presence of twice the normal amount of PLP1. New techniques such as multiplex ligation-dependent probe amplification (MLPA) and microarray-based comparative genomic hybridization (CGH) can also be used for diagnosis. In 15%–25% of affected individuals, a point mutation in the PLP1 protein coding region is observed. Amino acid substitution is the most frequently seen type of mutation, although other splicing abnormalities and nonsense mutations, insertions, and deletions may also occur. A method such as direct nucleotide sequencing is used to detect such mutations. Deletion of the entire length of the PLP1 gene is rare (£2%). Deletions can be detected by any of the methods mentioned above. The genetic diagnosis of PMD requires the use of a combination of detection methods, considering the diverse variety of mutations possible.
(2) Pelizaeus-Merzbacher-Like Disease 1 (PMLD1, HLD2, OMIM#311601)
Disease concept
Although its clinical symptoms are indistinguishable from those of PMD, this disease is differentiated from PMD and known as Pelizaeus-Merzbacher-like disease because it is not caused by a mutation in PLP1. This rare disorder includes cases of girls that were formerly diagnosed with PMD. In some affected individuals with autosomal recessive inheritance, it is caused by a mutation in the gene encoding gap junction protein C2 (GJC2).2
Clinical symptoms and test results
Nystagmus is apparent soon after birth and motor developmental retardation is noticeable before 1 year of age, after which pyramidal tract, cerebellar, and basal ganglia symptoms appear in the same manner as in PMD. Prolongation of the central nervous conduction time is evident in the auditory brainstem response, and T2-weighted cranial MRI reveals signs including diffuse hyperintensity of the white matter. These clinical symptoms and test results are very similar to those seen in PMD. Maximum motor development is better than that seen in PMD, with 12 out of 33 reported cases being capable of walking independently. However, all the above individuals began to regress before reaching 10 years of age and lost the ability to walk without support by then. Affected individuals who developed mild cases of spastic paraplegia have also been reported.
Causative genes
Defects in the GJC2 gene (also known as Cx47 and GJA12) were reported in 2004, demonstrating that this condition also differs genetically from PMD.2 However, GJC2 mutations are not present in some individuals with PMLD, suggesting that other genes may also be involved. The known mutations are all located within coding regions, and may cause the loss of function of the gap junction on the cell membrane of oligodendrocytes.3 These mutations are detected by nucleotide sequencing for genetic diagnosis.
(3) Hypomyelination with atrophy of the basal ganglia and cerebellum (HABC, HLD6, OMIM%612438)
Disease concept
This disease is characterized by progressive atrophy of the basal ganglia (most pronounced in the heads of the caudate nuclei and the putamen) in addition to congenital white matter hypomyelination. HABC was first described in 2002, and was established as a new disease in 2007.4,5
Clinical symptoms
No abnormalities, including nystagmus, are evident at birth, in most cases. Maximum motor function is achieved by the age of 3 years, when affected individuals are able to walk with support. Their development begins to regress before they are 10 years old, and they lose the ability to walk with support over a period of several years. Spasticity and cerebellar ataxia are not obvious at birth but develop gradually, and the appearance of extrapyramidal tract symptoms such as athetosis, dystonia, and rigidity is characteristic of this disease. Although mental retardation ranges from moderate to severe, affected individuals attempt to communicate with smiles and other facial expressions. Almost 50% of the affected individuals exhibit sensory deafness, microcephaly, and short stature.
Test results
Cranial MRI reveals diffuse T2-weighted hyperintensity. Although the brainstem is hypointense, the pyramidal tract is characteristically hyperintense. The white matter volume is low and the cerebral ventricles are enlarged. The hyperintense cerebral and cerebellar white matter on T2-weighted images gradually becomes hypointense, reflecting the loss of myelin. Atrophic lesions are present in the cerebellum, and these are particularly severe in the vermiform process. The thalamus and the globus pallidus are preserved, but the putamen is small and eventually disappears. The caudate nuclei also gradually atrophy.
Causative gene
TUBB4A was reported as one of the genes associated with these diseases by Simons et al. in 2013.6 In addition, a mutation in this gene has also been reported to cause the autosomal dominant dystonia DYT4.7
(4) Chromosome 18q deletion syndrome (18qdel, OMIM#601808)
Disease concept
The 18q deletion syndrome is a chromosome aberration syndrome in which the terminal end of the long arm of chromosome 18 is lost. As the deleted region includes numerous genes, it causes a wide range of symptoms. Cerebral hypomyelination is caused by the deletion of the myelin basic protein (MBP) gene.8
Clinical symptoms
Affected individuals exhibit a diverse range of symptoms, including impaired growth, (particularly short stature), developmental retardation, hypotonia, impaired coordination, nystagmus, conductive hearing loss, seizures, microcephaly, facial midline hypoplasia, sunken eyes, blepharophimosis and carp-shaped mouth. The prognosis for survival is good. Important comorbidities associated with 18q deletion syndrome include ureteral reflux and urinary tract infection.
Test results
Cranial T2-weighted MRI reveals hyperintensity of the cerebral white matter varying in extent from hyperintensity of the entire cerebral white matter to scattered areas of hyperintensity. Hypointensity of the corpus callosum tends to be preserved. Blood tests often reveal IgA deficiency and low levels of secreted growth hormone. Some studies have reported normal peripheral nerve conduction velocity and visual evoked potentials. However, no study has described the auditory brainstem response yet.
Genetic diagnosis
Majority of reported cases contain the 18q21→qter long arm terminal deletion, and in three-quarters of affected individuals, this mutation is de novo (a mutation in the index case). Other reported cases include familial reciprocal balanced translocation, de novo translocation and familial inversion. G-band staining or FISH is used for diagnosis. The 18q22→q23 region on the chromosome is implicated in the symptoms of the disease. In affected individuals who exhibit cerebral white matter lesions, FISH analysis identifies the loss of one copy of the MBP gene located in 18q23. Delayed myelination is believed to occur due to haploinsufficiency of the MBP gene.
(5) Allan-Herndon-Dudley syndrome (AHDS, OMIM#300523)
Disease concept
AHDS is an X-linked mental retardation syndrome, the main symptoms of which include severe mental retardation, dysarthria, athetoid movement, hypotonia, and spastic paraplegia; MRI reveals delayed myelination starting at infancy. It is believed to be caused by impaired membrane transport of the thyroid hormone due to a defect in the solute carrier family 16, member 2 [SLC16A2; monocarboxylic acid transporter 8 (MCT8)] gene on Xq13.2.9 A recent study has shown that SLC16A2 (MCT8) mutations are present in approximately 10% of individuals with PMD.10
Clinical symptoms
Affected individuals exhibit so-called syndromic X-linked mental retardation, and show a wide spectrum of symptoms. The most severely affected individuals exhibit severe hypotonia, nystagmus, dystonia, and tetanic spastic paraplegia in addition to delayed motor and speech development. Symptoms presented by mildly affected individuals, male or female, start with hypotonia and gradually develop into spastic paraplegia, dysarthria, ataxic gait, chorea, and facial muscle pathology. No other secretory functional abnormalities other than that of the thyroid hormone are reported. No visceral malformations or hearing loss is observed.
Test results and imaging findings
Affected individuals exhibit abnormal thyroid hormone levels, in that the thyroid hormone tests reveal low T4 and high T3, while thyroid-stimulating hormone (TSH) is often at the upper limit of normal. Patients also show characteristic imaging findings; cranial MRI may show almost no abnormalities initially, but signs of hypomyelination such as cortical atrophy or diffuse hyperintensity on T2-weighted imaging gradually begin to appear. No study of the acoustic brainstem response has been reported in previous literature, although conduction velocity in the central nervous system is believed to decrease.
Genetic diagnosis
SLC16A2 (MCT8) mutations are identified by nucleotide sequencing or other methods. SLC16A2 is located in q13.2 on the X chromosome, and encodes a protein with 12 membrane-spanning domains. SLC16A2 (MCT8) is believed to be involved in the active transport of thyroid hormone across the membrane. Nonsense and missense mutations have been reported, and the severity of the condition varies according to the type of mutation. A case of translocation in the SLC16A2 (MCT8) domain in a girl child has also been reported. SLC16A2 (MCT8) mutation is suspected if the following symptoms are present: (1) X-linked spastic paraplegia with severely retarded psychomotor development, (2) impaired myelin formation on cranial MRI, and (3) low T4, high T3, and TSH at the upper limit of normal on thyroid hormone tests.
(6) Mitochondrial heat-shock protein 60 chaperonopathy (MitCHAP60, HLD4, OMIM #612233)
Disease concept
This form of congenital cerebral hypomyelination is caused by an abnormal mitochondrial chaperonin protein Hsp60. The gene responsible for this abnormality is the autosomal recessive heat shock protein family D (Hsp60) Member 1 (HSPD1)gene, located on chromosome 2q33.1. The only cases reported so far have all come from an extended family in Israel in which repeated intermarriage between blood relatives has occurred.11
Clinical symptoms
According to ten cases reported in members of the family described above, hypotonia, nystagmus and psychomotor retardation are noticeable during the first three months of life. Severe spasticity, developmental delay, and regression are apparent. About 50% of the affected individuals also suffer from epilepsy. Poor food intake leads to malnutrition and impaired growth. Most of the affected individuals die of aspiration pneumonia or sudden death due to an unknown cause before the age of 20. Some severely affected individuals die during the first year or two of life, while those who survive more than 2 years exhibit progressive spastic paralysis and contracture of the limbs. Differences in phenotype are apparent within and between families.
Test results
MRI reveals diffuse hyperintensity of the cerebral and cerebellar white matter on T2-weighted images, with no myelination whatsoever. Thinning of the corpus callosum, enlarged cerebral ventricles and atrophy of the brainstem and cerebellum are also evident. In the auditory brainstem response, wave I is delayed and waves II–V are lost.
Genetic diagnosis
Diagnosis is made through DNA nucleotide sequencing of HSPD1. In the extended family described above, the condition appears in individuals homozygous for the D29G mutation, and heterozygous carriers are asymptomatic. Another HSPD1 mutation causes type 13 spastic paraplegia (SPG13; OMIM#605280), which is an autosomal dominant disorder.
(7) Salla disease (SD, OMIM#604369)
Disease concept
SD is an autosomal recessive disorder characterized by the accumulation of sialic acid in lysosomes. MRI reveals the same diffuse white matter lesions seen in congenital cerebral hypomyelination. The causative gene is SLC17A5 (6q14-q15). The same gene mutation is known to cause infantile sialic acid storage disorder (ISSD, OMIM#269920), which is fatal in infancy and a slow-progressing, mild disorder during adulthood.
Clinical symptoms
Most affected individuals come from the Salla region of Finland. Their symptoms include developmental delay, developmental regression, poor physical growth, ataxia, nystagmus, hypotonia, spasticity, and epilepsy.
Test results
Elevated urinary free sialic acid (N-acethylneuraminic acid [NANA]) is a useful marker for diagnosis. In rare cases, NANA is not elevated in urine, but all affected individuals have elevated NANA in cerebrospinal fluid. MRI reveals a hypomyelinating pattern of extensive hyperintensity of the white matter on T2-weighted imaging, similar to that seen in PMD. Magnetic resonance spectroscopy (MRS) shows high N-acetylaspartate(NAA), however, this is because NANA and NAA are indistinguishable on 1.5-T magnetic resonance devices. This result, therefore, actually reflects elevated NANA and is of diagnostic value. Peripheral nerve conduction velocity is diminished in around half of affected individuals, but visual evoked potentials and the auditory brainstem response are generally both normal.
Genetic diagnosis
Most cases of SD are caused by an R39C founder mutation in the SLC17A5 gene, and no case has yet been reported from Japan. Recent studies have identified a small number of mutations other than R39C that cause SD. It is conjectured that the molecular pathology of the cerebral lesions may comprise impaired nerve conduction due to the impaired glutamate/aspartate transport function of defective SLC17A5.
(8) Diffuse cerebral hypomyelination with cerebellar atrophy and hypoplasia of the corpus callosum (HCAHC)
Disease concept
This new disease concept was proposed by Japanese researchers in 2009.12 Cerebellar atrophy and hypoplasia of the corpus callosum occur in addition to hypomyelination of the central nervous system. There is no atrophy of the basal ganglia.
Clinical symptoms
All three of the affected individuals reported to date were capable of walking independently at age 1–3, but exhibited ataxic gait, nystagmus, bradylalia, pyramidal tract symptoms and mild to moderate psychomotor developmental retardation after they entered their teens. Athetosis was not present in any case, but two of the three patients exhibited dystonia.
Test results
Peripheral nerve conduction velocity, visual evoked potentials, and the auditory brainstem response are all normal. MRI reveals diffuse T2 hyperintensity and equivalent or milder T1 hyperintensity. Hypoplasia of the corpus callosum and cerebellar atrophy (particularly in the cortex) are present. Although cerebral atrophy is not initially present, it is seen at the point at which clinical regression becomes evident.
Genetic diagnosis
Saitsu et al. reported in 2011 that this disease is associated with mutations in the POLR3A and POLR3B genes, which encode the RNA polymerase III protein.13
(9) Other unclasssifiable disorders
Type 2: Congenital hypomyelination of the central and peripheral nervous systems
(1) Hypomyelination and congenital cataract (HCC, HLD5, OMIM#610532)
Disease concept
The combination of cerebral hypomyelination and congenital cataract as a single disease entity was first reported as a new form of autosomal recessive congenital cerebral hypomyelination in 2006.14 It is a rare condition, with only 10 affected individuals reported so far.
Clinical symptoms
Cataract is noticeable within 1 month of birth, and although the affected individuals gain the ability to hold up their heads and sit up by themselves, developmental retardation becomes apparent when they are still unable to walk at over 1 year of age. They are able to walk with assistance at 2 years of age, but are never able to walk independently. Regression is slow, and 9 out of the 10 affected individuals had lost the ability to walk by the time they reached adulthood. All the patients exhibited extrapyramidal tract symptoms, and although pathological reflexes were present, tendon reflexes characteristically decreased. Microcephaly was present in 9 out of the 10 cases, and peripheral nerve symptoms in the form of muscular weakness and atrophy of the distal leg muscles were also present. Basal ganglia symptoms (such as dystonia and athetosis) have not been described. Mental retardation is mild to moderate, and convulsions other than febrile convulsions were seen to occur in 2 of the affected individuals.
Test results
Peripheral nerve conduction velocity was low in 9 of the 10 affected individuals, and peripheral nerve biopsy revealed a decrease in myelinated fibers, with hypomyelination visible under electron microscopy. MRI reveals diffuse T2 hyperintensity and equivalent or milder T1 hyperintensity. However, in some periventricular areas, there are regions that are strongly hyperintense on T2-weighted images but hypointense on T1-weighted images, suggesting that the water content of white matter may be higher in these areas.
Genetic diagnosis
Mutations are identified by DNA nucleotide sequencing of the causative gene, FAM126A (DRCTNNB1A) (7p15.3). FAM126A is associated with Wnt/β-catenin signaling and has also been implicated in the differentiation of oligodendrocytes and the regulation of the synthesis of phosphatidylinositol 4-phosphate in membranes.15
(2) Ataxia, delayed dentition, and hypomyelination (ADDH, HLD7, OMIM 612440)
Disease concept
This form of cerebral hypomyelination is characterized by progressive ataxia with oligodontia, adontia, and other forms of hypodontia.16 Its time of onset ranges from infancy to early childhood, and its symptoms are all progressive. It is a rare condition, with all the affected individuals reported to date belonging to fewer than 20 families. The causative gene has yet to be identified. Most cases are sporadic, but it has sometimes been reported to have occurred in blood relatives; its mode of inheritance may be either autosomal recessive or autosomal dominant, suggesting that it is genetically heterogeneous. Other symptoms such as mental retardation and hypogonadotropic hypogonadism may also be present.
Clinical symptoms
Development in infancy is usually normal. However, motor developmental delay becomes obvious from the point at which individuals learn to walk independently. Affected individuals whose development has been normal during infancy start to exhibit obvious ataxia in their early teens, although the age at which symptoms become overtly apparent varies somewhat between families. Pyramidal tract symptoms are present in some individuals but not others. Oligodontia, adontia, and other forms of hypodontia (mainly missing incisors) become noticeable during infancy. Other obvious symptoms include hypotonia and impaired growth. Some affected individuals may not display secondary sexual characteristics as a result of hypogonadotropic hypogonadism, but the great variation in the age of the reported cases means that it is unclear whether or not this endocrine characteristic is always present in all cases. Mental retardation ranges from none to moderate. Owing to the small number of reported cases, the prognosis remains unknown.
Test results
Cranial MRI reveals hypomyelination as diffuse hyperintensity of the cerebral white matter on T2-weighted images as well as cerebellum atrophy. Thinning of the corpus callosum is apparent. MRS also has diagnostic value, with characteristic findings of elevated myoinositol and decreased NAA and choline in the cerebral white matter. Hypodontia is determined by panoramic X-ray to identify missing teeth.
Genetic diagnosis
The causative gene and its locus have yet to be identified.
(3) Peripheral demyelinating neuropathy, central dysmyelinating leukodystrophy, Waardenburg syndrome, and Hirschsprung disease (PCWH, OMIM#609136)
Disease concept
This is a rare disease characterized by the dysgenesis not only of oligodendrocytes, but also of Schwann cells, melanocytes, intestinal ganglion cells and other neural crest-derived cells.17 Clinically, it combines four different syndromes, including hypomyelination of the central nervous system and demyelinating neuropathy in the peripheral nervous system, in addition to Waardenburg syndrome and Hirschsprung disease. The gene associated with this disorder is the autosomal dominant SOX10 gene, , with most cases being sporadic due to a sudden mutation.18
Clinical symptoms
Most affected individuals require surgical treatment for Hirschsprung disease in the immediate postnatal period. In terms of neurological symptoms, in the most severe cases, there is almost no myelination of either the peripheral or central nervous system, and the affected individuals die at an early age. Moderately severe cases exhibit psychomotor developmental retardation and hypotonia, with around half of them also exhibiting spastic quadriplegia and demyelinating neuropathy. In mild cases, mild motor developmental retardation and demyelinating neuropathy are apparent. Waardenburg syndrome causes partial hypopigmentation of the iris, hair and skin, as well as sensorineural deafness.
Test results
Cranial MRI reveals diffuse hyperintensity of the cortical white matter of varying degrees of severity on T2-weighted images.18 In severe cases, cortical dysplasia resulting in atrophy of the brainstem, cerebellum, and cerebral cortex is apparent. In mild cases, only minute hyperintensities are present in the periventricular white matter. Peripheral nerve conduction velocity changes also vary with severity.
Genetic diagnosis
In most cases, the cause is a point mutation in the coding region of the SOX10 gene, which is identified by DNA nucleotide sequencing. This usually occurs as a novel mutation in affected infants. Cases of the deletion of genome regions including the SOX10 gene have also been reported.
Other unclassifiable disorders
5. Summary
Since there are almost no specific biochemical markers for congenital cerebral hypomyelination, its diagnosis must necessarily depend on diagnostic MRI, genetic analysis and extensive clinical evaluation. In this article, we have described the classification and concepts of 11 different disorders, although there are still a few affected individuals who do not fit into any of these categories. Unfortunately, it is not trivial to determine whether these cases may represent new diseases caused by unknown mutations, or are caused by known gene defects, by using the genetic analysis systems currently available for use in Japan. The only way to carry out further investigations of the disease concepts and pathologies of congenital cerebral hypomyelination is to gather information pertaining to individual cases as they arise. We hope that the diagnostic criteria summarized in this article will be of use in clinical settings, and intend to continue to revise them in light of the diversity of phenotypes of known disorders as well as the identification of new diseases.
The diagnostic criteria described in this article are based on the content of the diagnostic criteria reported in the FY2009 Ministry of Health, Labour and Welfare (MHLW) Scientific Grant-in-Aid for Scientific Research Report (with some revisions and additions). This study was carried out with support from the MHLW Scientific Grant-in-Aid Research to Overcome Intractable Diseases Research Project for the Diagnosis and Treatment of Congenital Cerebral Hypomyelination, and with the approval of the Joint Research Support Project of the Japanese Society of Child Neurology.
References
- Inoue, K. PLP1-related inherited dysmyelinating disorders: Pelizaeus-Merzbacher disease and spastic paraplegia type 2. Neurogenetics 6, 1-16, doi:10.1007/s10048-004-0207-y (2005).
- Uhlenberg, B. et al. Mutations in the gene encoding gap junction protein a12 (connexin 46.6) cause Pelizaeus-Merzbacher-like disease. Am J Hum Genet 75 (2004).
- Orthmann-Murphy, J. L., Enriquez, A. D., Abrams, C. K. & Scherer, S. S. Loss-of-function GJA12/Connexin47 mutations cause Pelizaeus-Merzbacher-like disease. Mol Cell Neurosci 34, 629-641 (2007).
- van der Knaap, M. S. et al. Hypomyelination with atrophy of the basal ganglia and cerebellum: follow-up and pathology. Neurology 69, 166-171, doi:10.1212/01.wnl.0000265592.74483.a6 (2007).
- van der Knaap, M. S. et al. New syndrome characterized by hypomyelination with atrophy of the basal ganglia and cerebellum. AJNR. American journal of neuroradiology 23, 1466-1474 (2002).
- Simons, C. et al. A de novo mutation in the beta-tubulin gene TUBB4A results in the leukoencephalopathy hypomyelination with atrophy of the basal ganglia and cerebellum. American journal of human genetics 92, 767-773, doi:10.1016/j.ajhg.2013.03.018 (2013).
- Hersheson, J. et al. Mutations in the autoregulatory domain of beta-tubulin 4a cause hereditary dystonia. Ann Neurol 73, 546-553, doi:10.1002/ana.23832 (2013).
- Gay, C. T. et al. Magnetic resonance imaging demonstrates incomplete myelination in 18q- syndrome: evidence for myelin basic protein haploinsufficiency. Am J Med Genet 74, 422-431 (1997).
- Dumitrescu, A. M., Liao, X. H., Best, T. B., Brockmann, K. & Refetoff, S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet 74, 168-175 (2004).
- Vaurs-Barriere, C. et al. Pelizaeus-Merzbacher-Like disease presentation of MCT8 mutated male subjects. Ann Neurol 65, 114-118 (2009).
- Magen, D. et al. Mitochondrial hsp60 chaperonopathy causes an autosomal-recessive neurodegenerative disorder linked to brain hypomyelination and leukodystrophy. Am J Hum Genet 83, 30-42 (2008).
- Sasaki, M. et al. Diffuse cerebral hypomyelination with cerebellar atrophy and hypoplasia of the corpus callosum. Brain Dev 31, 582-587 (2009).
- Saitsu, H. et al. Mutations in POLR3A and POLR3B encoding RNA Polymerase III subunits cause an autosomal-recessive hypomyelinating leukoencephalopathy. American journal of human genetics 89, 644-651, doi:10.1016/j.ajhg.2011.10.003 (2011).
- Zara, F. et al. Deficiency of hyccin, a newly identified membrane protein, causes hypomyelination and congenital cataract. Nat Genet 38, 1111-1113 (2006).
- Baskin, J. M. et al. The leukodystrophy protein FAM126A (hyccin) regulates PtdIns(4)P synthesis at the plasma membrane. Nature cell biology 18, 132-138, doi:10.1038/ncb3271 (2016).
- Wolf, N. I. et al. Leukoencephalopathy with ataxia, hypodontia, and hypomyelination. Neurology 64, 1461-1464 (2005).
- Inoue, K., Tanabe, Y. & Lupski, J. Myelin deficiencies in both the central and peripheral nervous system associated with a SOX10 mutation. Ann Neurol 46, 313-318 (1999).
- Inoue, K. et al. Molecular mechanism for distinct neurological phenotypes conveyed by allelic truncating mutations. Nat Genet 36, 361-369 (2004).
Figure 1.
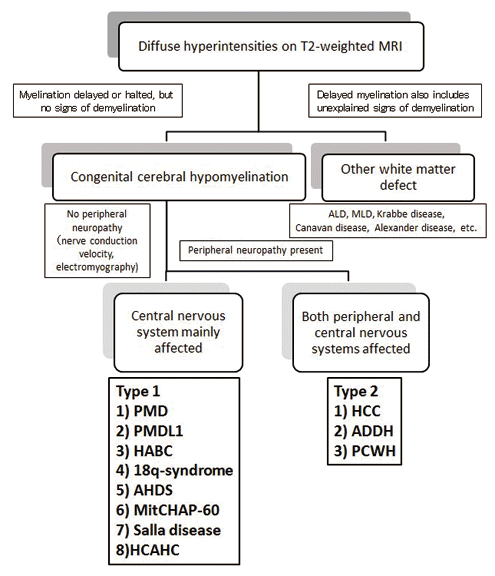
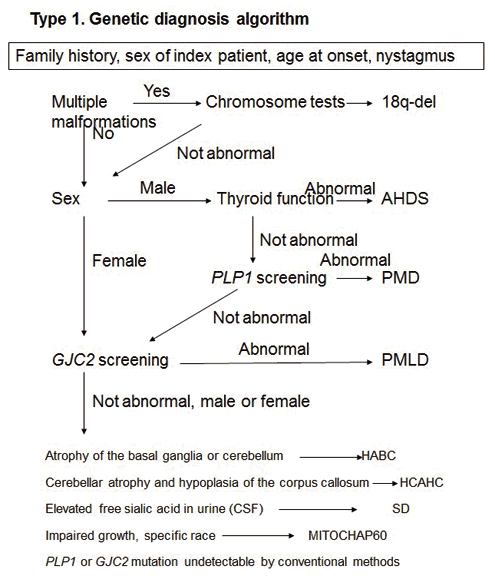
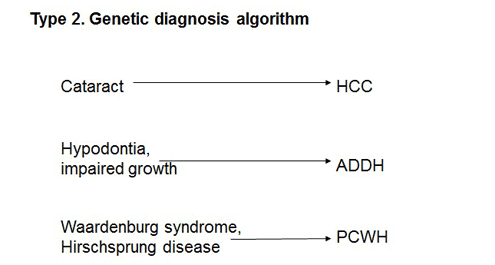
Table 1. Classification of congenital cerebral hypomyelination
| Disease name | OMIM | Abbreviation | HLD classification | Type of inheritance | Locus | Causative gene | |
| Type 1: Diseases with congenital hypomyelination mainly of the central nervous system | |||||||
| (1) | Pelizaeus-Merzbacher disease | #312080 | PMD | HLD1 | X-linked recessive | Xq22 | PLP1 |
| (2) | Pelizaeus-Merzbacher-like disease 1 | #608804 | PMLD1 | HLD2 | Autosomal recessive | 1q42.13 | GJC2 |
| (3) | Hypomyelination with atrophy of the basal ganglia and cerebellum | %612438 | HABC | HLD6 | Unknown | Unknown | Unknown |
| (4) | 18q deletion syndrome | #601808 | 18q- DEL |
Chromosomal | 18q22- qter |
MBP | |
| (5) | Allan-Herndon-Dudley syndrome | #300523 | AHDS | X-linked recessive | Xq13.2 | SLC16A2 | |
| (6) | Mitochondrial Hsp60 chaperonopathy | #612233 | MITCH AP60 |
HLD4 | Autosomal recessive | 2q33.1 | HSPD1 |
| (7) | Salla disease | #604369 | SD | Autosomal recessive | 6q13 | SLC17A5 | |
| (8) | Diffuse cerebral hypomyelination with cerebellar atrophy and hypoplasia of the corpus callosum | Not yet established | HCAHC | Unknown | Unknown | Unknown | |
| Type II: Diseases with congenital hypomyelination of the central and peripheral nervous systems | |||||||
| (1) | Hypomyelination and congenital cataract | #610532 | HCC | HLD5 | Autosomal recessive | 7p15.3 | FAM126A |
| (2) | Ataxia, delayed dentition, and hypomyelination | %612440 | ADDH | HLD7 | Unknown | Unknown | Unknown |
| (3) | Peripheral demyelinating neuropathy, central dysmyelinating leukodystrophy, Waardenburg syndrome, and Hirschsprung disease | #609136 | PCWH | Autosomal dominant | 22q13 | SOX10 | |
| HLD: Hypomyelinating leukodystrophy. Some of the diseases included in congenital cerebral hypomyelination are classified as HLD in the Online Mendelian Inheritance in Man (OMIM) database, and we have therefore followed this in our classification. | |||||||