Early Diagnosis Method
Development of Early Screening Methods for glucose transporter1 deficiency
Diagnosing GLUT1 Deficiency Syndrome (Glut1 deficiency) often requires comprehensive testing, including characteristic clinical findings, decreased CSF glucose/blood glucose ratio, and genetic testing, which can be time-consuming. To begin treatment before chronic brain dysfunction occurs, we considered the need for a simple and minimally invasive screening method.
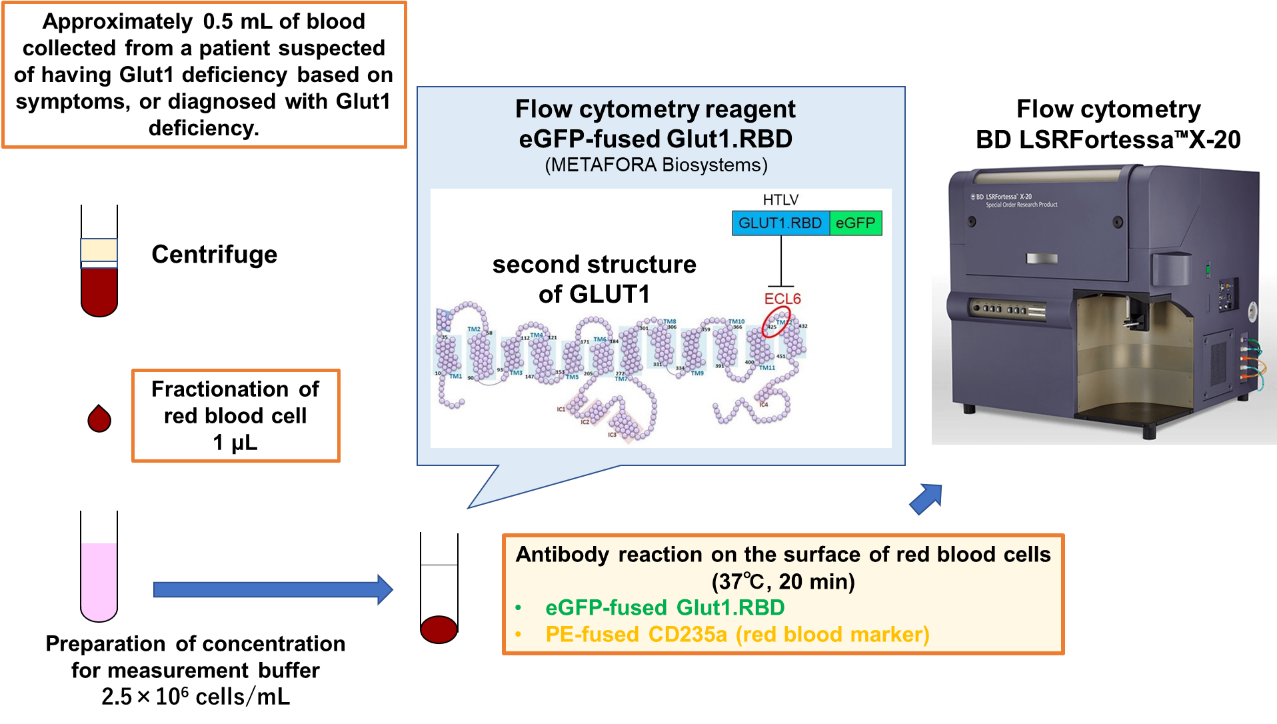
The method used was to measure the expression level and structural changes of GLUT1 on the erythrocyte membrane by flow cytometry using 1 ml of erythrocyte fraction centrifuged from approximately 0.5 ml of blood collected from patients who had been genetically diagnosed with Glut1 deficiency or were suspected of having Glut1 deficiency based on clinical symptoms. The reagent used for flow cytometry was Glut1.RBD (METAFORA), which combines the fluorescent dye GFP with the receptor binding domain of HTLV, which recognizes the three-dimensional structure of the extracellular domain of GLUT1.
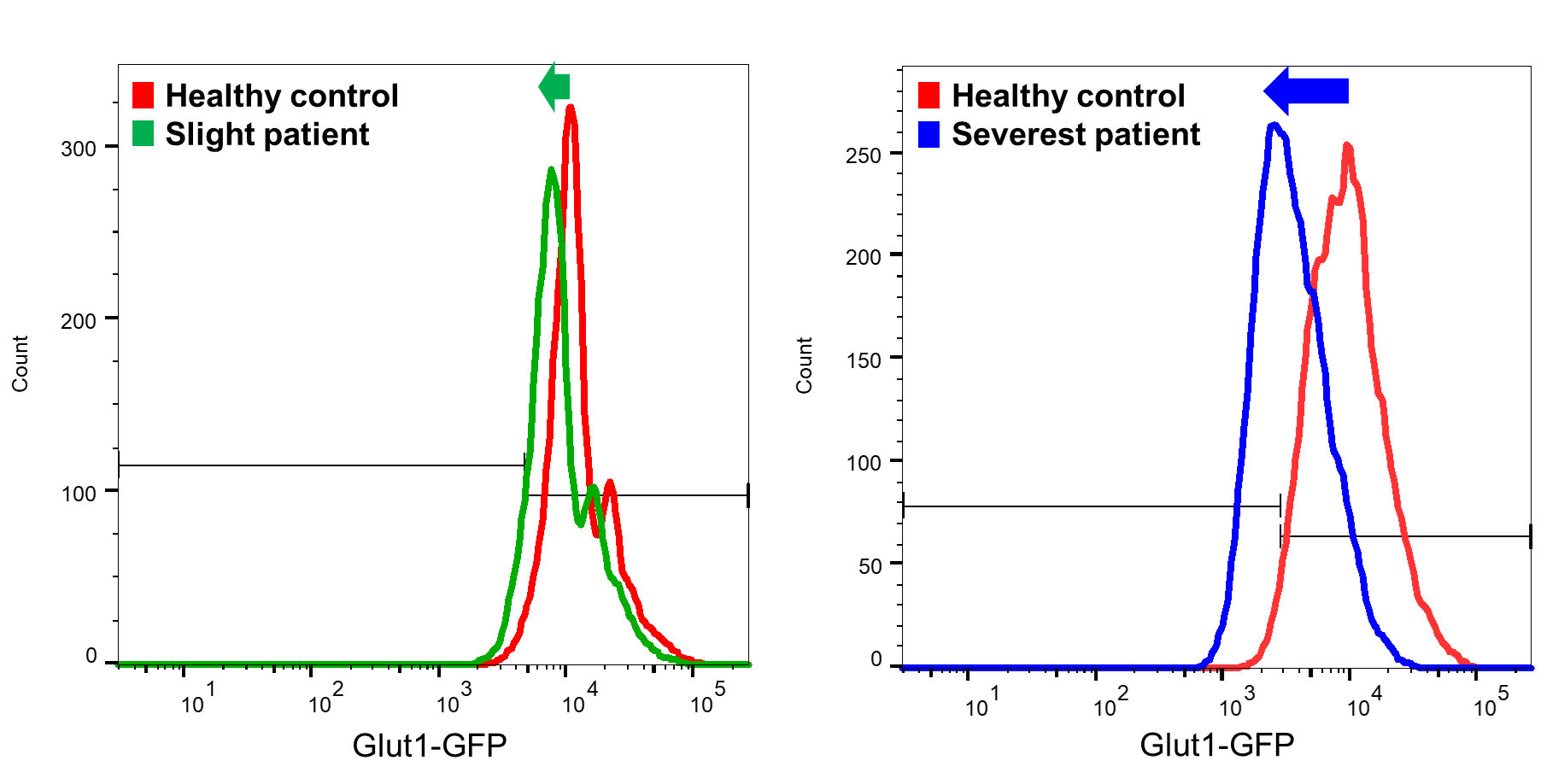
The results showed that the GLUT1 fluorescence intensity of 13 healthy subjects was 96.5±0.61%, while that of 13 Glut1 deficiency patients was 82.0±3.0%, a significant decrease. One patient with clinical symptoms like Glut1 deficiency but without genetic abnormality showed no decrease in fluorescence intensity. When classified by the severity of intellectual disability before starting the ketogenic diet, the GLUT1 fluorescence intensity of 4 patients with mild to moderate intellectual disability was 82.2±4.7%, and that of 7 patients with severe to profound intellectual disability was 77.5±3.6%, significantly decreased compared to healthy subjects (Figure shows a representative histogram. The graph shifts to the left as the severity increases). Based on these results, it is possible that the flow cytometry method can be used to detect severe Glut1 deficiency early without causing neurological damage and with less burden than cerebrospinal fluid testing. We are currently increasing the number of cases and continuing to investigate this.
Reference
- Mol Genet Metab Rep. 2023; 34: 100954.


