Treatment Development
Development of GLUT1 therapy using AAV
Previous research has demonstrated that AAV vectors (carriers), artificially created based on AAV, can efficiently introduce target genes into the body, enabling the synthesis of target proteins over the long term.
AAV vectors can be used as therapeutic vectors by removing the gene for a virus-derived protein and loading a therapeutic gene into the remaining space. The investigator and a pharmaceutical company conducting collaborative research incorporated the SLC2A1 gene into an AAV vector to treat GLUT1 deficiency, and injected GT0006X, which activates GLUT1 in the body, to a mouse model of GLUT1 deficiency. They succeeded in improving motor function, one of the symptoms of GLUT1 deficiency. In addition, the activity of the SLC2A1 gene was sustained for a long period of time in the GT0006X-injected mice, and no particular side effects were observed. Therefore, GT0006X is expected to show similar effectiveness in patients’ GLUT1 deficiency.
In this clinical trial, GT0006X is an investigational product that will be injected intrathecally to you.
GT0006X has not been used in humans before. Another gene therapy product that uses an AAV vector is a regenerative medicine product called onasemnogene abeparvovec, which has already been approved for the treatment of spinal muscular atrophy. The GT0006X uses the same type of AAV vector as the one used in onasemnogene abeparvovec, but it is slightly different in that some of the amino acids on the outside of the vector have been modified.
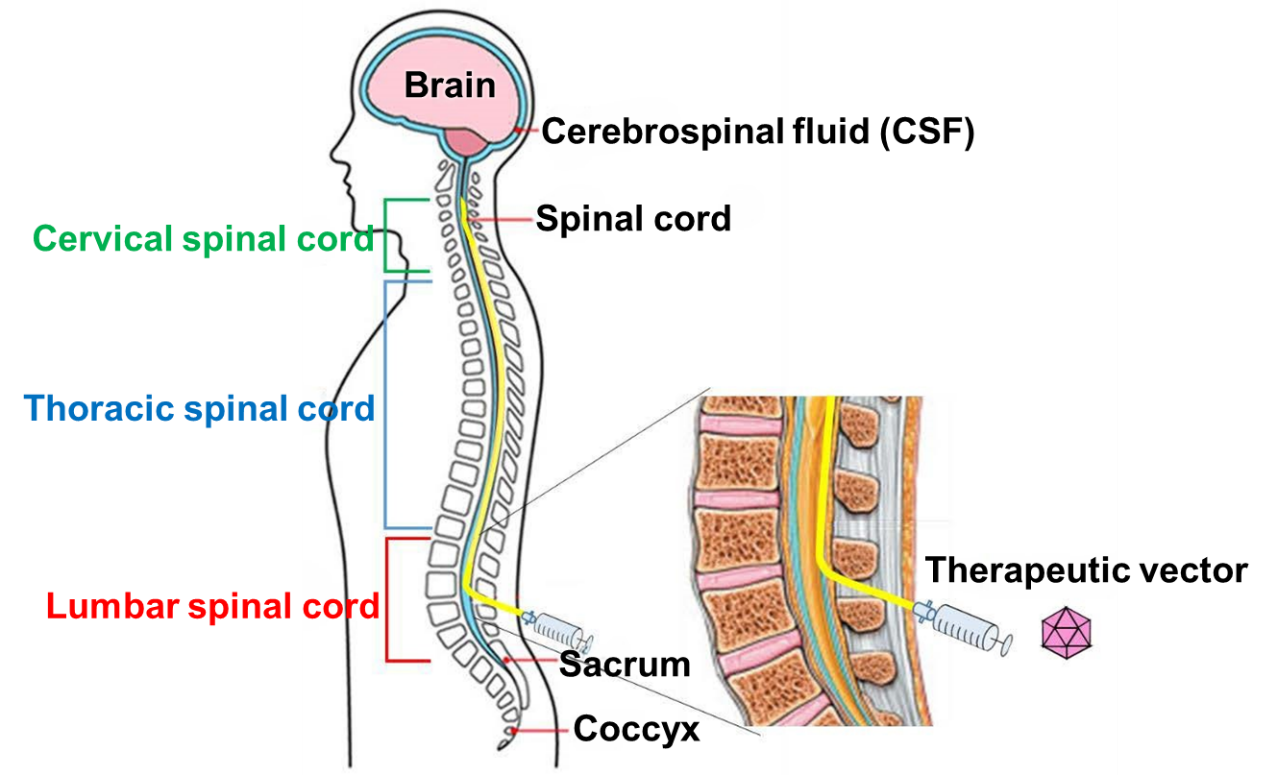
To inject GT0006X, an injection needle (intrathecal injection needle) is inserted between the back and lumbar vertebrae and into the subarachnoid space (spinal cavity). Next, a catheter is inserted through the needle from the upper thoracic vertebrae to the height of the cervical vertebrae (cisterna magna). After injection, the catheter is removed (Figure 2).
The surgery and injection will be performed in an operating room. Local anesthesia will be used during injection. The procedure leading up to injection may be subject to change depending on the situation at the time.
The estimated time required for surgery and injection, including anesthesia, is about 1 to 2 hours. GT0006X will only be injected once during the clinical trial period.
The catheter used in this trial is not a government-approved medical device but was developed for injecting GT0006X or similar therapeutic vectors and has not been used in humans before. A similar approved medical device (catheter for injecting other drugs into the spinal cord from the lumbar vertebrae) exists but is designed for continuous drug injection. The catheter for this trial was developed specifically for injecting therapeutic vectors like GT0006X.
The GT0006X dosage is set in two stages to determine the safe and effective amount. Initially, three patients will receive a low dose (2 × 10¹² vg/kg), followed by another three patients receiving a higher dose (6 × 10¹² vg/kg). Before injecting the higher dose, 3 to 20 mL of cerebrospinal fluid will be collected to avoid a sudden increase in fluid volume.
To ensure safety, GT0006X will not be injected into the next patient until at least one month has passed since the first patient was injected GT0006X. The investigator will review the patient's data (test results, etc.) for one month after the injection of GT0006X to determine whether there have been any problems with the patient's progress or any serious side effects and will only inject the drug to subsequent patients if it is deemed to be safe.
In addition, before the clinical trial proceeds to the higher dose in the second phase, a third-party committee called the Independent Safety Assessment Committee will comprehensively review the data from the three patients who received the lower dose in the first phase to determine whether there are likely to be any problems with injecting the dose in the second phase. Only if it is determined that there are no problems can the clinical trial proceed to the higher dose in the second phase.
This Independent Safety Assessment committee is composed of experts and physicians who are not involved with this clinical trial, ensuring that the review is conducted from an impartial standpoint.


