University Hospital Clinical Trial Alliance (UHCT Alliance)
Procedures for clinical trials (1): from consultation to site selection
UHCT Alliance Office
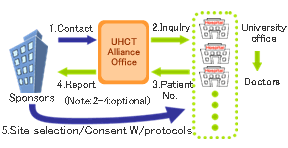
Sponsors can use the UHCT Alliance for both domestic and multi-national clinical trials. We deal with both types equally. If sponsors are considering selecting the members of the UHCT Alliance as trial sites, please contact the Alliance Office by phone or e-mail either directly or through one of our members. Detailed information about the product to be investigated is not necessary for the initial consultation. Alliance Office staff will serve you under a confidentiality agreement and inform you about how to apply for a clinical trial through the Alliance.
|
Inquiry to members of the UHCT Alliance
The UHCT Alliance Office will provide information about your clinical trial to the offices of our members. Our members in turn will ask doctors in their hospitals about the possibility of implementation, as well as the number of eligible patients. If a sponsor prepares a questionnaire about its trial, the offices of our members will ask their doctors to answer it as soon as possible. We can reply to such questionnaires without being told the company names or the details of the product candidate. An initial inquiry about the availability of eligible patients can be answered by UHCT Alliance office within a week. Sponsors can select a trial site after obtaining the answers from each member and can contact a principal investigator at the selected site thereafter.
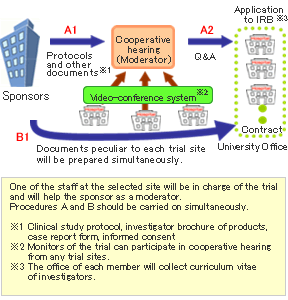
Cooperative hearing using video-conference system
The UHCT Alliance has introduced a video-conference system to facilitate cooperative hearing of the protocols of clinical trials. Before the hearing, the staff of the selected site will check the protocol, and common questions will be prepared by one of our staff. Sponsors can explain the protocols and answer the questions at the cooperative hearing, and our members can share the information provided. Because the Alliance has a standardized form for obtaining informed consent, sponsors do not have to prepare various versions of it. Sponsors can save time and money.
One of the staff at the selected site will be in charge of the trial and will help the sponsor as a moderator of the cooperative hearing, adjust differences of opinion among the trial sites, and guide the sponsor throughout the trial.