Introductory Course (registration is required to attend)
- Subjects are announced from time to time.
- If you wish to enroll, please contact us.
- You can also register for a single lecture.
- Points are awarded in accordance with the attendance.
- Completion upon earning 20 points.
| No. | Subject | Schedule | Points |
|---|---|---|---|
| 1 | Overview of Clinical Study Design | Year-round | |
| 2 | Overview of Drug Development & Pharmacology | Year-round | |
| 3 | Clinical Researcher Training Curriculum | Every Friday (12:00 PM - 12:45 PM) |
1 |
| 4 | Study visit to Clinical Research Center | 2 days (irregular dates) | 7 |
| 5 | Management Seminar Lecturer: Yan Hao Chairman & CEO, EPS Holdings, Inc. |
May 18, 2015 | 2 |
| 6 | Lecture by Kazuhiko Toyama CEO, Industrial Growth Platform Inc. |
May 20, 2015 | 2 |
| 7 | Internship Report, second half of fiscal 2014 | May 25, 2015 | 1 |
| 8 | Special lecture by Andrew Thompson (CEO & Co-founder, Proteus Digital Health) |
May 30, 2015 | 2 |
| 9 | Seminar by Dr. Randall Bateman, MD | June 9, 2015 | 2 |
| 10 | Course on medical device venture (4th open seminar) | June 22, 2015 | 1 |
| 11 | Introductory course on drug development for cranial nerve disease | Aug. 6, 2015 | 4 |
| 12 | Off-campus seminar by Toshiba Medical Systems | Sept. 1, 2015 | 3 |
| 13 | Introductory course on drug development for (2nd seminar) cranial nerve disease | Sept. 1, 2015 | 4 |
| 14 | Introductory course on drug development for (3nd seminar) cranial nerve disease | Sept. 4, 2015 | 4 |
| 15 | Science Communication Workshop Instructor: Satomi Tsuboko |
Sept. 9, 2015 | |
| 16 | Science Communication Workshop Instructor: Prof. John Larry |
Sept. 10, 2015 | |
| 17 | Chemical compound screening workshop (Kanto region) | Sept. 10, 2015 | 4 |
| 18 | Special lecture on Developing New Drugs Using Big DataⅠEmerging Drugs & Diagnostics Development Program | Sept. 15, 2015 | 2 |
| 19 | Integrated Medical ManagementⅡ Guest lecturer: Hiroshi Otake |
Sept. 30, 2015 | |
| 20 | Emerging Drugs & Diagnostics Development Program Guest lecturer: Hirotatsu Kojima |
Oct. 1, 2015 | |
| 21 | Special lecture on Developing New Drugs Using Big DataⅡEmerging Drugs & Diagnostics Development Program |
Oct. 6, 2015 | 2 |
| 22 | Fun, Pain and Dilemma of Developing New Drugs | Oct. 8, 2015 | 1 |
| 23 | Management seminar by Yasuhiko Ishikawa Executive Vice President, Solasto Corporation |
Oct. 28, 2015 | 2 |
| 24 | 4th Symposium on Medical Innovation Initiative, The University of Tokyo | Oct. 29, 2015 | 5 |
| 25 | Drug Development from Viewpoint of Academia | Oct. 30, 2015 | 1 |
| 26 | Internship Program Reporting SessionⅠ | Nov. 20, 2015 | 3 |
| 27 | Community Organizing Workshop | Nov. 24, 2015 | 3 |
| 28 | Fiscal 2015, Medical Industry Innovation Forum | Nov. 25, 2015 | 3 |
| 29 | Special lecture on Developing New Drugs Using Big Data Ⅲ Emerging Drugs & Diagnostics Development Program | Nov. 26, 2015 | 2 |
| 30 | Introductory course on drug development for (4th seminar) cranial nerve disease | Nov. 27, 2015 | 2 |
| 31 | Leadership Development for the 21st Century | Nov. 30, 2015 | |
| 32 | Off-campus seminar by Olympus Corporation | Dec. 1, 2015 | 5 |
| 33 | Course on medical device venture (5th open seminar) | Dec. 21, 2015 | 1 |
| 34 | Internship Program Reporting Session Ⅱ | Feb. 1, 2016 | 2 |
| 35 | 2016 Advanced Medical Seeds Development Forum The University of Tokyo Hospital |
Feb. 2, 2016 | 4 |
| 36 | Seminar on Conducting Clinical Tria | Feb. 24, 2016 | 4 |
| 37 | Seminar on Professional life and Career Development of Japanese Researchers Active Overseas | March 17, 2016 | 2 |
| 38 | Project for a Bridge of Innovation Between Silicon Valley and Japan:Action Plan of the University of Tokyo and Stanford University | March 29, 2016 | 3 |
| 39 | Round table discussion with Japanese Students at Stanford University on Research Life and Career Development | March 31, 2016 | 1 |
Core Course (Admission required)
- The course is completed upon earning 10 credits.
- Minimum of 4 credits from lectures and 2 credits from applied training are required.
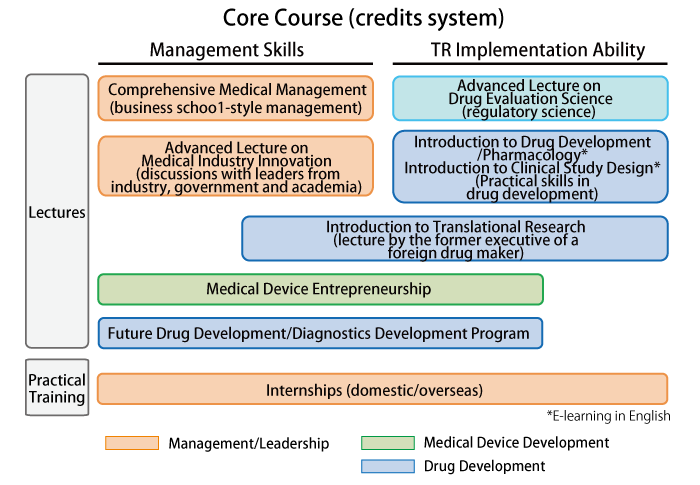
(Lectures scheduled to start in 2015)
Subject Details for Lectures to Be Held
| Subject | Comprehensive Medical Management I, II |
|---|---|
| Held on | Summer & winter semesters; Wednesdays; 6th period (6:45 PM – 8:30 PM) |
| Lecturer | Yoshihiro Shimomura |
| Credits | 2 each |
| Description |
|
| Subject | Introduction to Translational Research |
|---|---|
| Held on | Summer semester; Thursdays; 6th period (6:45 PM – 8:30 PM) |
| Lecturer | Masuhiro Kato |
| Credits | 2 |
| Description |
|
| Subject | Advanced Lecture on Pharmaceutical Evaluative Science |
|---|---|
| Held on | Winter semester; Mondays; 5th period (4:00 PM – 5:30 PM) |
| Lecturer | Shunsuke Ono |
| Credits | 2 |
| Description |
|
| Subject | Advanced Lecture on Medical Industry Innovation |
|---|---|
| Held on | Winter semester; held on irregular days (8-9 times total) (6:00 PM – 9:00 PM) |
| Lecturer | Hiromichi Kimura |
| Credits | 2 |
| Description |
|
| Subject | Introduction to Drug Development/Pharmacology, Introduction to Clinical Study Design |
|---|---|
| Held on | Throughout the year (e-learning in English) |
| Lecturer | The Clinical Research Support Center |
| Credits | 1 each |
| Description |
|
| Subject | Clinical Researcher/Specialist Training Curriculum |
|---|---|
| Held on | Throughout the year (e-learning in English) |
| Lecturer | The Clinical Research Support Center |
| Credits | 1 |
| Description |
|
| Subject | Medical Device Entrepreneurship (Advanced Level) |
|---|---|
| Held on | Summer semester; Tuesdays; 6th period (6:45 PM – 8:30 PM) |
| Lecturer | Yujiro Maeda |
| Credits | 2 |
| Description |
|
| Subject | Future Drug Development/Diagnostics Development Program (Advanced Level) |
|---|---|
| Held on | Winter semester; Thursdays; 6th period (6:45 PM – 8:30 PM) |
| Lecturer | Akiko Kishi, Hiroko Kimura |
| Credits | 2 |
| Description |
|
| Subject | Internships |
|---|---|
| Held on | Throughout the year |
| Lecturer | TBD by host |
| Credits | 2 (a period of more than two weeks) or 1 (a period of less than two weeks) |
| Description |
|