Guidelines on Hereditary Leukodystrophies
CQ 3-8. Pol III-related leukodystrophies (HDL7, OMIM #607694; HDL8, OMIM #614381; HDL11, OMIM #616494): hypomyelination, hypodontia, and hypogonadotropic hypogonadism (4H) syndrome; ataxia, delayed dentition, and hypomyelination (ADDH); tremor-ataxia with central hypomyelination (TACH); leukodystrophy with oligodontia (LO); and hypomyelination with cerebellar atrophy and hypoplasia of the corpus callosum (HCAHC)
Pol III–related leukodystrophies are autosomal recessive inherited diseases caused by POLR3A, POLR3B, or POLR1C mutations. Clinically, their basic symptoms are progressive gait disturbance due to spasticity or cerebellar ataxia and tremors; they also exhibit dental anomalies (hypodontia, delayed eruption, and abnormal tooth alignment) and hypophysial hypogonadism (delayed or absent sexual maturation). Magnetic resonance imaging reveals hypomyelination with cerebellar atrophy and hypoplasia of the corpus callosum. At this point, symptomatic therapy is provided.
Disease concept
Pol III–related leukodystrophy is typically difficult to diagnose, even if overall cerebral white-matter hypoplasia is evident on cranial magnetic resonance imaging (MRI). Sasaki et al. coined the term "hypomyelination with cerebellar atrophy and hypoplasia of the corpus callosum" for diseases that exhibit this combination of findings.1) Their clinical symptoms are known to include motor dysfunction (such as spasticity, cerebellar ataxia, and tremors), dental anomalies (such as hypodontia, delayed eruption, and abnormal tooth alignment), and hypophysial hypogonadism (delayed or absent sexual maturation), and several syndromes have been reported based on a combination of imaging findings and clinical symptoms: hypomyelination, hypodontia, and hypogonadotropic hypogonadism (4H) syndrome;2) ataxia, delayed dentition, and hypomyelination;3) tremor-ataxia with central hypomyelination;4) and leukodystrophy with oligodontia.5) The responsible genes (POLR3A, POLR3B, and POLR1C) have been identified,6-9) and these syndromes shown to represent a series of phenotypical differences that have been given the general name of Pol III–related leukodystrophies. 10)
Epidemiology
The incidence is unknown, but over 150 cases have been reported to date.
Etiology and pathophysiology
The POLR3A and POLR3B genes encode the subunits (RPC1 and RPC2) that form the core of the RNA polymerase III (POLR3) complex. Three-dimensional model analysis of this complex has shown that the reported mutations should affect POLR3 activity. POLR3 transcribes the genes that encode very large amounts of low-molecular-weight RNA, including tRNA and 6SrRNA. It is thought that insufficient amounts of this low-molecular-weight RNA may cause dysmyelination, but its detailed mechanism is unknown. POLR1C is a subunit of both POLR1 and POLR3, and the reported mutations prevent POLR3 formation and inhibit its transport to the nucleus.
Clinical symptoms
Motor dysfunction, dental anomalies, and hypogonadism are the three main clinical symptoms. In a study of 105 patients (43 with POLR3A mutations, 62 with POLR3B mutations),11) mean age at onset was 4.3 years for POLR3A and 3.4 years for POLR3B. Approximately half (52%) of the patients exhibited developmental delay, while 19 (4 POLR3A, 15 POLR3B) were unable to walk. However, in 10 cases, onset occurred after 10 years of age. Most patients exhibited intention tremors, dysmetria, gait ataxia, abnormal smooth pursuit eye movement, and gaze-evoked nystagmus. Restricted vertical vision, evident in 20%, was usually an early symptom. Most patients exhibited mild to moderate intellectual disability, with many slowly regressing in their teens. Epilepsy was present in 19% but responded well to drug treatment. Infection-associated regression occurred in 53% of cases. Dental anomalies such as hypodontia, delayed eruption, and abnormal dentition were seen in 87%, while delayed puberty and amenorrhea were present in 81% of patients with POLR3A mutations and 69% of those with POLR3B mutations. About half of patients (51%) were of short stature, and half of those patients in whom it was measured exhibited growth hormone deficiency. POLR3A mutations are considered clinically more serious than POLR3B mutations.11) Over 60% of patients with POLR3B mutations were able to walk with assistance by age 20, whereas in those with POLR3mutations, the rate dropped from 40% at age 20 to <10% among those aged 30 and over. POLR3A mutations also have a worse survival prognosis (see below).
Among the 8 reported cases of POLR1C mutations, neurological abnormalities were present in 8 of 8 patients, myopia in 4 of 8, and dental anomalies in 3 of 8.9)
Investigations
Cranial MRI of all patients with POLR3A and POLR3B mutations reveals overall diffuse faint hyperintensity (hypomyelination) of the cerebral white matter on T2-weighted imaging together with cerebellar atrophy and thinning of the corpus callosum, but the basal ganglia are spared. The ventrolateral thalamus, optic radiation, globus pallidus, and dentate nucleus are hypointense on T2-weighted imaging. Cerebellar atrophy is reportedly more severe in patients with POLR3B mutations than in those with POLR3A mutations, but hypomyelination is comparatively milder.12) The extent of myelination may be associated with the fact that the clinical symptoms of POLR3B mutations are milder than those of POLR3A mutations. Hypomyelination and thinning of the corpus callosum were present in all 8 reported patients with POLR1C mutations, while cerebellar atrophy was evident in 5 of 8.
A study of cerebral pathology13) (36 men with POLR3A mutations) found severe loss of myelin and oligodendrocytes, with moderate axon loss and mild astrocyte and microglial proliferation. The presence of macrophages engulfing apparently normal oligodendrocytes suggested an immunological mechanism. Astrocyte proliferation was evident in layers I and II of the cerebral cortex.
Genetic diagnosis
Heterozygous mutations of the POLR3A, POLR3B, and POLR1C genes have been reported.
Treatment and care
At this point, only symptomatic therapy is provided.10) Neurological symptoms (spasticity and epilepsy) are managed by medical treatment and rehabilitation. Comprehensive treatment and care with the participation of dentists, ophthalmologists, endocrinologists, and rehabilitation specialists is desirable.
Prognosis
In a study of 105 patients (43 with POLR3A mutations, 62 with POLR3B mutations), 8 deaths were recorded (at age 8–36 years). Only 1 of those 8 patients carried a POLR3B mutation, suggesting that POLR3A mutations may have a worse prognosis.11)
Figure.
Pol III–associated leukodystrophy (POLR3B mutation)
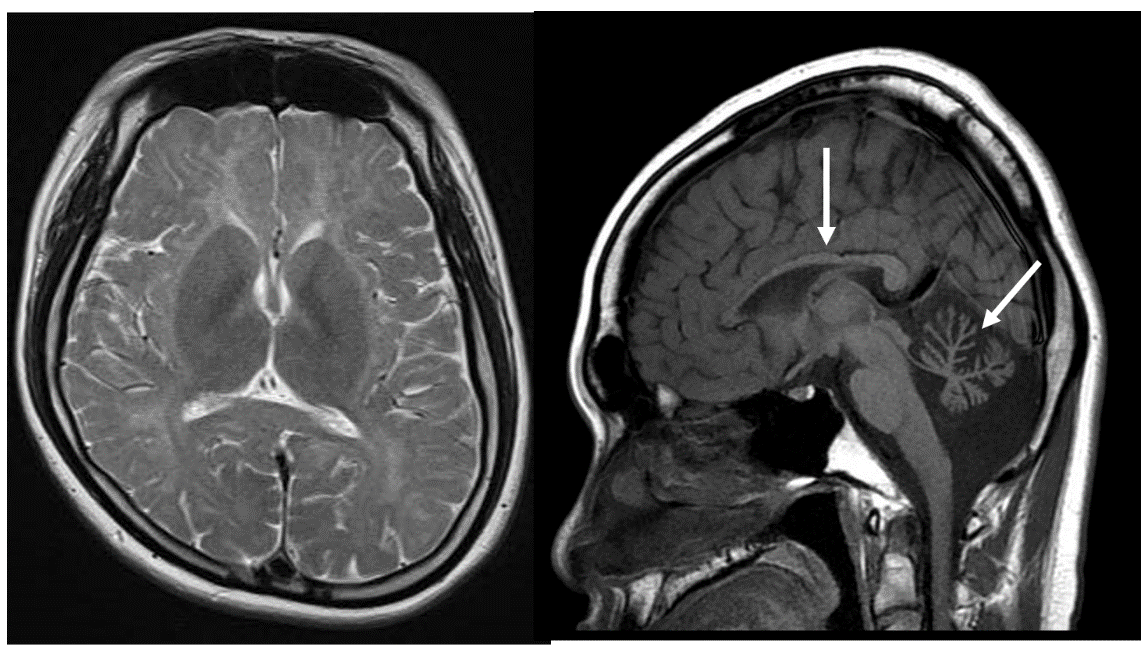
A male in his 20s with developmental delay and hypogonadism was found to carry a POLR3B mutation. On T2-weighted imaging, the cerebral white matter was hyperintense, while cerebellar atrophy and hypoplasia of the corpus callosum (arrow) were present.
References (evidence levels in parentheses)
- Sasaki M, Takanashi J, Tada H, et al. Diffuse cerebral hypomyelination with cerebellar atrophy and hypoplasia of the corpus callosum. Brain Dev 2009; 31: 582-587.(5)
- Timmons M, Tsokos M, Asab MA, et al. Peripheral and central hypomyelination with hypogonadotropic hypogonadism and hypodontia. Neurology 2006; 67: 2066-2069.(5)
- Wolf NI, Harting I, Boltshauser E, et al. Leukoencephalopathy with ataxia, hypodontia, and hypomyelination. Neurology 2005; 64: 1461-1464.(5)
- Bernard G, Thiffault I, Tetreault M, et al. Tremor-ataxia with central hypomyelination (TACH) leukodystrophy maps to chromosome 10q22.3-10q23.31. Neurogenetics 2010; 11: 457-464.(5)
- Atrouni S, Darazé A, Tamraz J, et al. Leukodystrophy associated with oligodontia in a large inbred family: fortuitous association or new entity? Am J Med Genet A 2003; 118A: 76-81.(5)
- Bernard G, Chouery E, Putorti ML, et al. Mutations of POLR3A encoding a catalytic subunit of RNA polymerase pol III cause a recessive hypomyelinating leukodystrophy. Am J Hum Genet 2011; 89: 415-423.(5)
- Tetreault M, Choquet K, Orcesi S, et al. Recessive mutations in POLR3B, encoding the second largest subunit of pol III, cause a rare hypomyelinating leukodystrophy. Am J Hum Genet 2011; 89: 652-655.(5)
- Saitsu H, Osaka H,Sasaki M, et al: Mutations in POLR3A and POLR3B encoding RNA polymerase III subunits cause an autosomal recessive hypomyelinating leukoencephalopathy. Am J Hum Genet 2011; 89: 644-651.(5)
- Thiffault I, Wolf NI, Forget D, et al. Recessive mutations in POLR1C cause a leukodystrophy by impairing biogenesis if RNA polymerase III. Nat Commun 2015; 6: 7623.(5)
- Bernard G, Vanderver A. Pol III-related leukodystrophies. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Ledbetter N, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews(R) [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2017.
2012.(6) - Wolf NI, Vanderver A, van Spaendonk RML, et al. Clinical spectrum of 4H leukodystrophy caused by POLR3A and POLR3B mutations. Neurology 2014; 83: 1898-1905.(4b)
- Takanashi J, Osaka H, Saitsu H, et al. Different patterns of cerebellar abnormality and hypomyelination between POLR3A and POLR3B mutations. Brain Dev 2014; 36: 259-263.(5)
- Vanderver A, Tonduti D, Bernard G, et al. More than hypomyelination in Pol-III disorder. J Neuropathol Exp Neurol 2013; 72: 67-75.(5)
PubMed search
- Pol[All Fields] AND III-related[All Fields] AND leukodystrophy[All Fields] 8 results
- POLR3A[All Fields] AND leukodystrophy[All Fields] 26 results
- POLR3B[All Fields] AND leukodystrophy[All Fields] 19 results
- POLR1C[All Fields] AND leukodystrophy[All Fields] 4 results