We will conduct expeditious and solid clinical research and clinical trials.
We will conduct safe and efficient trials through collaboration and cooperation between university hospitals with outstanding achievements, to supply drugs or medical devices that have high medical needs.
We will actively cooperative in developing drugs to address unmet needs of intractable and rare diseases by taking advantage of the university hospital's characteristics.
We will conduct safe and efficient trials through collaboration and cooperation between university hospitals with outstanding achievements, to supply drugs or medical devices that have high medical needs.
We will actively cooperative in developing drugs to address unmet needs of intractable and rare diseases by taking advantage of the university hospital's characteristics.
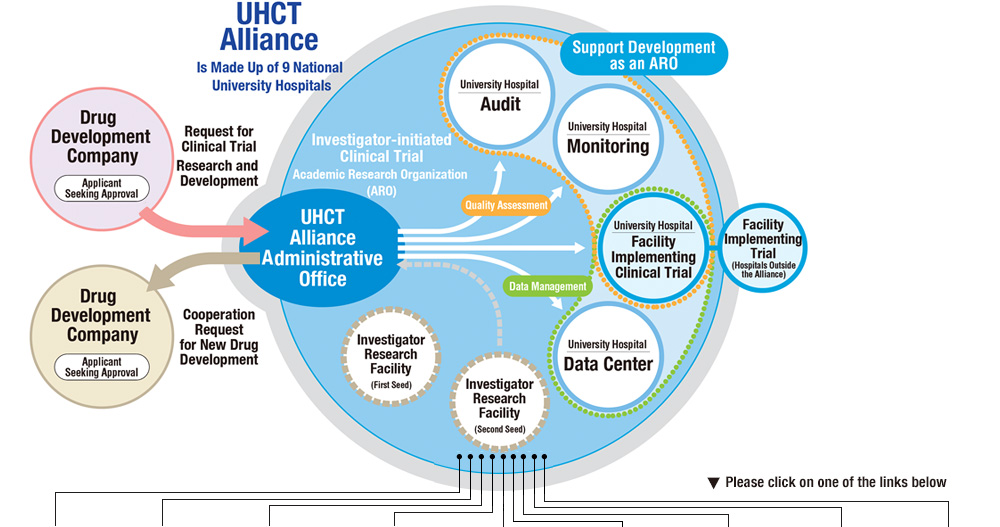
We aim to function as an ARO to coordinate investigator-initiated clinical trials.
University conducts research in various areas and has the potential of developing new drugs by nurturing the seeds of research. We will foster alliance network as an ARO by sharing resources and experience that becomes difficult for one member university, thereby step up as an organization that will contribute to new drug development.
University conducts research in various areas and has the potential of developing new drugs by nurturing the seeds of research. We will foster alliance network as an ARO by sharing resources and experience that becomes difficult for one member university, thereby step up as an organization that will contribute to new drug development.

University Hospital Clinical Trial Alliance (UHCT Alliance)
| Gr9 | External Relations Committee |
As Alliance administrative office we will conduct external management activities such as acceptance of industry-sponsored trials thereby facilitate coordination among member universities.

Clinical Research Promotion Division
| Gr1 | Seeds Development Committee |
Aims to explore and support nurturing of the drug and medical device seeds.
Propose environment building towards clinical application based on medical needs.

Center for Clinical Research
| Gr2 | Ethics Education Committee. Joint Ethical Review Committee Installation Working Group |
Aims to ensure that all clinical investigator receive high quality ethics education and
clinical trials are conducted correctly.
We will also consider the possiblity of installing joint ethics committee so that trials are implemented smoothly.

Clinical Research Support Center
| Gr3a | Researchers and Professional Staff Development Committee |
We will build educational program to train investigators and professionals to properly conduct clinical trials.
Also offer e-learning program and setup workshops.

Center for Translational Research
We will promote translational research so that seeds produced from basic research blossom into novel health care products.

Medical & Dental Hospital Bioscience Medical Research Center
| Gr4 | Clinical Research Coordinator (CRC) Liasion Committee |
We will develop Clinical Research Coordinator(CRC) education program to support academia-led clinical trials and organize forums intended for CRCs.

Clinical Research Center
| Gr3b | Committee for Nurturing Researchers & Professionals |
| Gr5 | Clinical Development Promotion Committee |
We will cultivate human resources to perform clinical trial monitoring within the university and promote exchange activities between the monitors.
We will examine and establish clinical trial system and implement trials through mutual cooperation between the personnel.
In addition, we will develop responsibility-sharing system and create price list for clinical trials.

Clinical Investigation and Research unit
| Gr6 | Case Series Study Committee |
We will cooperate in implementing targeted patients survey for clinical study and conduct survey on rare disease cases.
We will also connect hospital-hospital and hospital-clinic cooperation by setting up a patient referral system.

Clinical Research Promotion Center
Tsukuba Clinical Research & Development Organization
| Gr7 | Project Promotion and Quality Control Committee |
| Gr3a | Researchers and Professional Staff Development Committee |
We will carry out mutual enlightenment activities on the progress of collaborative projects and quality control thereby establishing a system to implement safe and efficient clinical trials.
Will foster professional staff for the development of medical device.

Clinical Research Center
| Gr8 | Public Relations and Enlightenment Committee |
We will renew PR medium and rebuild our website into a public-relations conscious site and develop strategy for effective utilization of PR medium.
We will reconsider information intended for researchers and promote resource development.
Furthermore, we will promote the steady-state availability of information to the general public by developing information provision strategy.
Copyright © University Hospital Clinical Trial Alliance, All Rights Reserved.