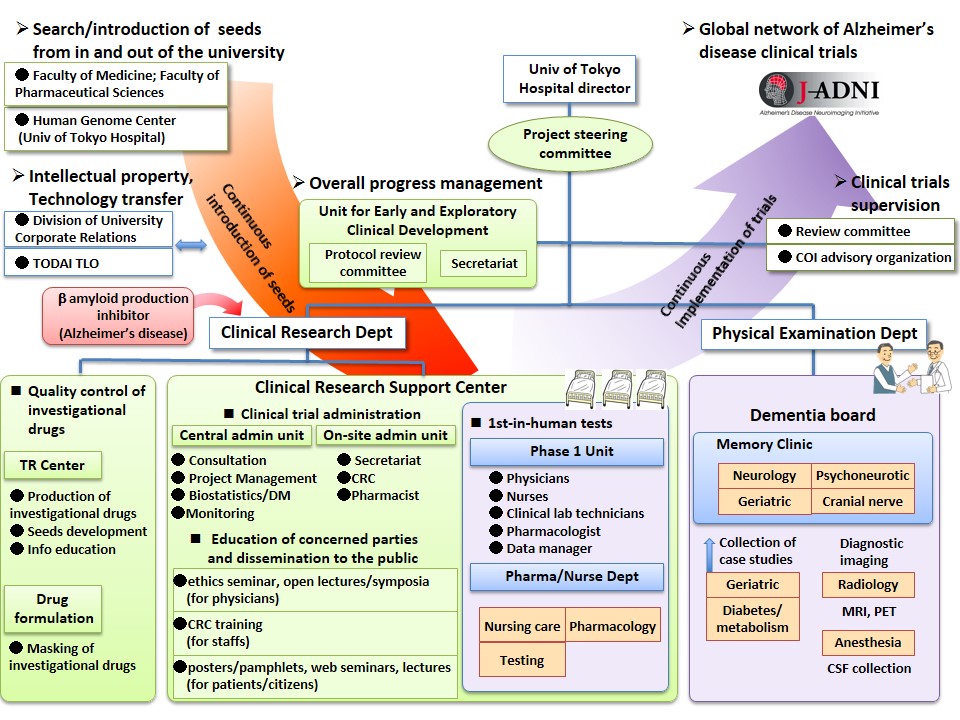
Overview
This program aims to construct a system to implement not only first-in-human (glossary) phase 1 studies about drug candidates for neurological and psychiatric illnesses, but also proof-of-concept clinical trials (glossary) for verifying the efficacy of such substances on human beings.
Target illnesses
- Alzheimer's disease
Having one of the most advanced aging societies in the world, Japan is said to have more than 4.3 million dementia patients, with more than 60% of those patients believed to be suffering from Alzheimer's disease. There are drugs that improve the symptoms of Alzheimer's disease, but there are still no disease modifying drugs. We are therefore carrying out clinical trials for disease modifying drug candidates for Alzheimer's disease to verify their efficacy.
- Multiple system atrophy
Multiple system atrophy is a rare disease with about 12,000 patients in Japan, and despite advancing symptoms of nervous disorders such as cerebellar ataxia, there is yet no effective treatment to restrain the progression. The University of Tokyo is now carrying out clinical studies to verify the efficacy of drug candidates discovered through genome analyses of multiple system atrophy patients. The Unit provides supports to this research.
- Autism
Autism is a type of developmental disorder and it is believed to have a morbidity of more than 1 in 100 people. There are drugs for anxiety disorders that accompany autism but there is yet no effective drug for treating the core symptoms such as social communication disorders. We support the development of drugs that are efficacious in treating the core symptoms of autism.
- Other
There are many more unmet medical needs (disorders without appropriate drugs and treatment) other than those listed above. We will concentrate our efforts to meet those needs.
[Glossary]
- First in Human
- Drug development involves a process of first verifying the efficacy and safety of drugs with animal tests, to then move on to tests with human patients. This latter process is known as clinical trials. Clinical trials are divided into 3 phases. With phase 1 tests, the drug under test (except for anticancer drugs) is administered to healthy adult volunteers to study the pharmacokinetics and side effects. Since the drug is first administered to human beings rather than animals, these are known as "First-in-human tests".
- Proof of Concept
- In case a certain molecule is believed to be a drug development target, a hypothesis ('concept') is formulated that a certain substance that acts on the target may be a therapeutic agent for the disease. The hypothesis is then examined whether it proves directly or indirectly that the substance has therapeutic efficacy on patients through appropriate indexes. The efficacy and safety of drugs in development are proved through 3 phases of clinical trials, and the verification of the concept is carried out in the phase 2 tests involving a limited number of patients.